It is one of the noble gases with the stable noble gas configuration and are colourless and odourless.
What is the physical state of argon at room temperature.
Argon state of matter at room temperature is gaseous.
An example is the halogen element chlorine.
That is why it is able to be a part of the atmosphere.
Which from argon and chlorine is gas at room temp.
What state is argon at room temperature.
Argon is an element that is a gas at room temperature.
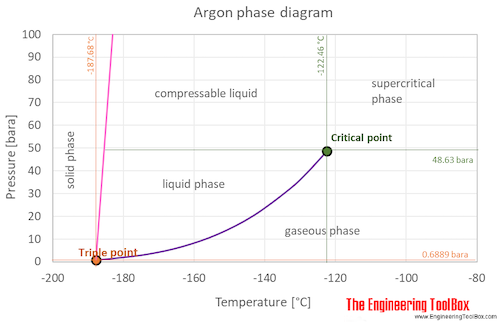
The curve between the critical point and the triple point shows the argon boiling point with changes in pressure.
Argon is chemically inert under most conditions and forms no confirmed stable compounds at room temperature.
Old documents and other things that are susceptible to oxidation can be protected by being stored in an atmosphere of argon.
Argon is one of the known 118 chemical elements which means it cannot be broken down further by chemical means.
The physical state of oxygen at room temperature is a gas.
That state of matter of an element may be predicted based on its phase diagram.
The state of matter at room temperature for the element europium is a liquid.
The physical state of maria argon is canada.
The physical state of petroleum at room temperature is liquid.
Is argon the element that is in gaseous state at room temperature.
Argon is colorless odorless nonflammable and nontoxic as a solid liquid or gas.
When pressure is controlled other pure elements may be found at room temperature.
Although argon is a noble gas it can form some compounds under various extreme conditions.
The argon phase diagram shows the phase behavior with changes in temperature and pressure.
Argon is a gas at room temperature.
While temperature is an easily controlled factor manipulating pressure is another way to cause a phase change.
However at low temperature and or high pressures the gas becomes a liquid or a solid.
The state of matter is a physical property.
Is state at room temperature a physical or chemical property.
What is europiums state of matter at room temperature.